The LipiDiDiet trial
LipiDiDiet (LDD) study is the first clinical trial to investigate the effects of a medical food in patients with prodromal Alzheimer's disease (AD), often referred to as "Mild Cognitive Impairment" (MCI), and considered early stage AD.
Nutricia is one of 19 partners in the LDD Consortium. Souvenaid® (containing the key nutrient combination "Fortasyn Connect®") was selected as the intervention product within the LDD study, on the basis of its results in an earlier EU project (LipiDiet, completed 2000-2005), and other early development work.
These results in patients with MCI support and extend findings from two previous randomised controlled trials of Souvenaid in patients with mild AD. We are pleased that this adds to the body of evidence for Souvenaid and we remain committed to ongoing and further clinical research.1,2
Visit the LipiDiDiet Consortium at http://www.lipididiet.eu
LipiDiDiet 3-year study results
Based on the results of Souvenir I and II, an independent European Commission funded a new trial focusing on the longer terms use of Souvenaid for memory and cognitive function in the very early stages of Alzheimer's disease - known as "prodromal AD" or "mild cognitive impairment" (MCI). With patients potentially followed up to 8 years, the LipiDiDiet study is the longest intervention trial in MCI.1
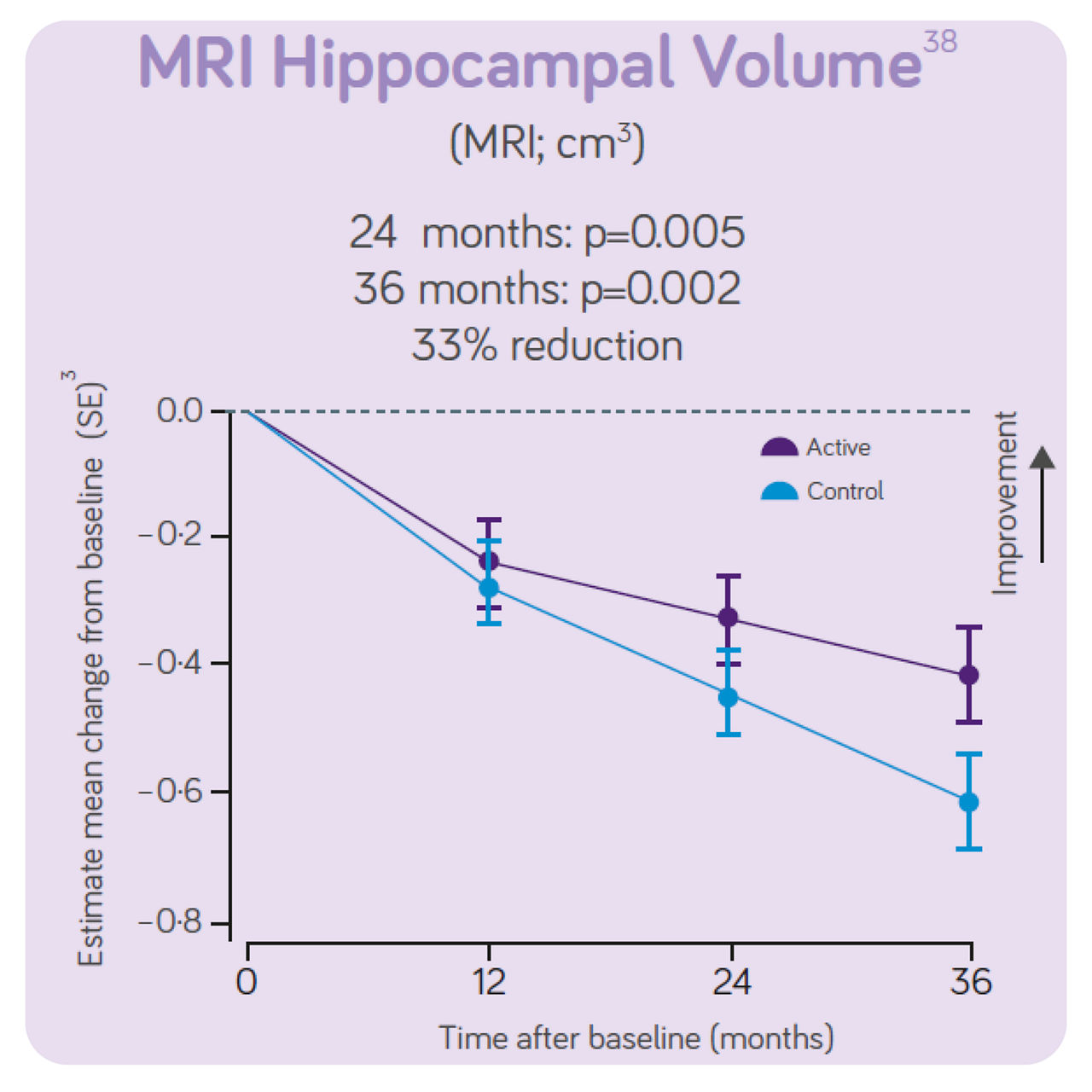
The trial concluded that, in people with prodromal Alzheimer's disease/MCI, the daily consumption of Souvenaid showed significant positive results (slowed decline) in cognitive function, thinking skills, memory, and brain atrophy.3
The clinical data also indicates:
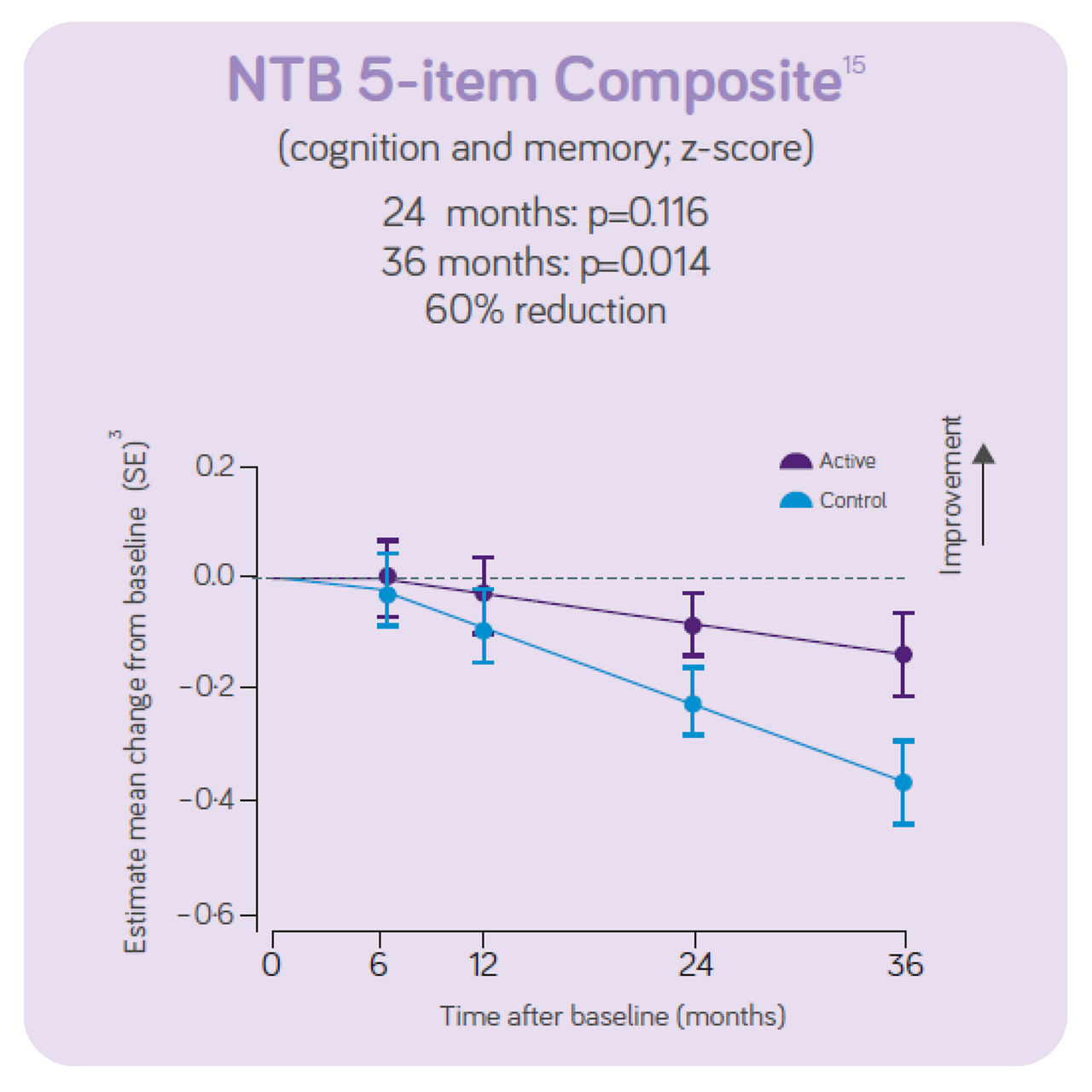
The decline in NTB 5-item (primary outcome on cognition and memory) showed a 60% reduction in decline in the active group vs the control group.
The rates of deterioration for hippocampal volume were 33% less in the active group than the control group.
Additional results also revealed rates of deterioration were 22% less in the active group than in the control group for whole brain volume.
With participants followed for up to 8 years, the LipiDiDiet trial is the longest intervention trial in prodromal AD. The results of this 3 year intervention with Fortasyn Connect (Souvenaid) show:
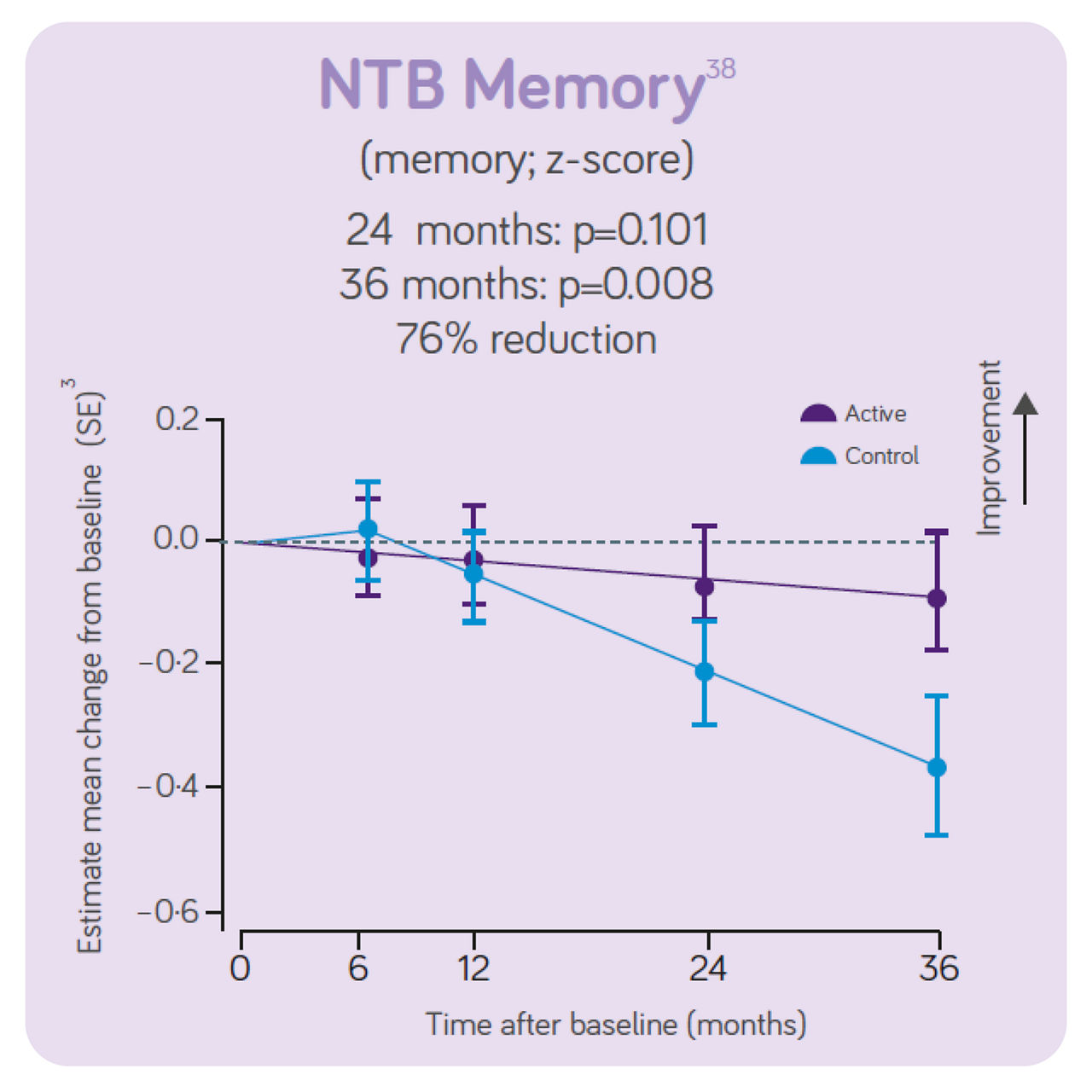
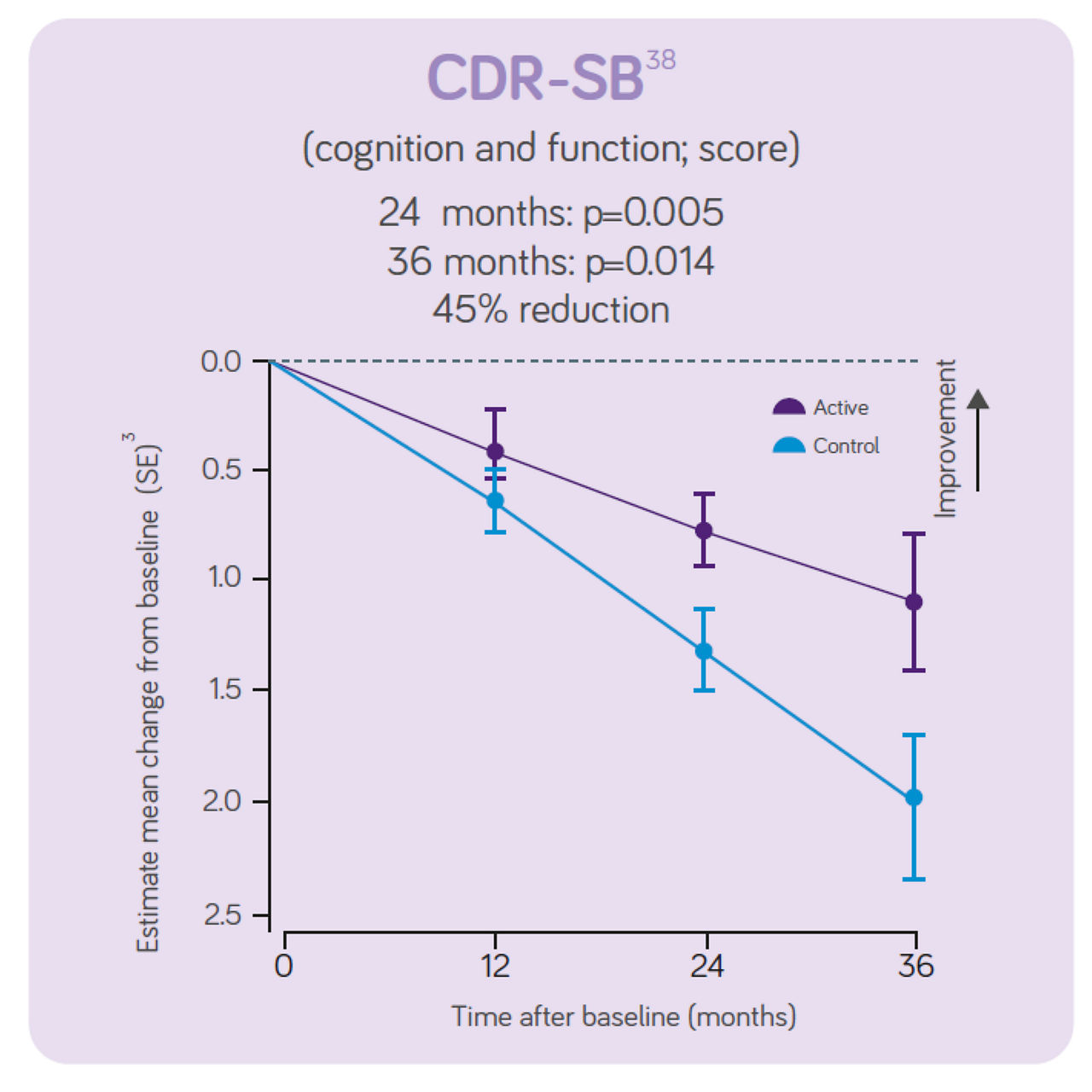
Sustained effects on CDR- SB, hippocampal volume and ventricular volume. Significant differences in NTB 5 -item, NTB memory and whole brain volume between groups. Increased benifits on all these outcomes over 3-year intervention. Good tolerance and safety profile maintained for 3 years in line with the 2 -year data indicating the intervention is most effective when started early. The 3 - year data indicates the intervention benefit increases with long-term use.
- Freund-Levi Y, Visser PJ, Kivipelto M, Wieggers RL, Hartmann T, Soinnen H. The LipiDiDiet study: rationale and study design. CtAD, San Diego, November 2011; J Nutr Health Aging, 2011; 15 (Suppl 1).
- Dubois, B., Feldman, H. H., Jacova, C., DeKosky, S. T., Barberger-Gateau, P., Cummings, J., … & Scheltens, P. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS–ADRDA criteria. The Lancet Neurology, 2007; 6(8): 734-746.
- Soininen H, Solomon A, Visser PJ, et al. 36-month LipiDiDiet multi-nutrient clinical trial in prodromal.
- Soininen H et al. 24 - month intervention with a specific multi-nutrient in people with prodromal Alzheimers's disease (LipiDiDiet): randomised, double blind controlled trial; Lancet Neurology 2017;16: 965-975