Importance of sensory alterations for cancer patients with taste alteration
The Journal of Supportive Care in Cancer published a study, showing that taste and smell alterations are common in patients receiving systemic antitumor therapy and that nutritional support specifically designed to better address sensory alterations, is appreciated in these patients.1

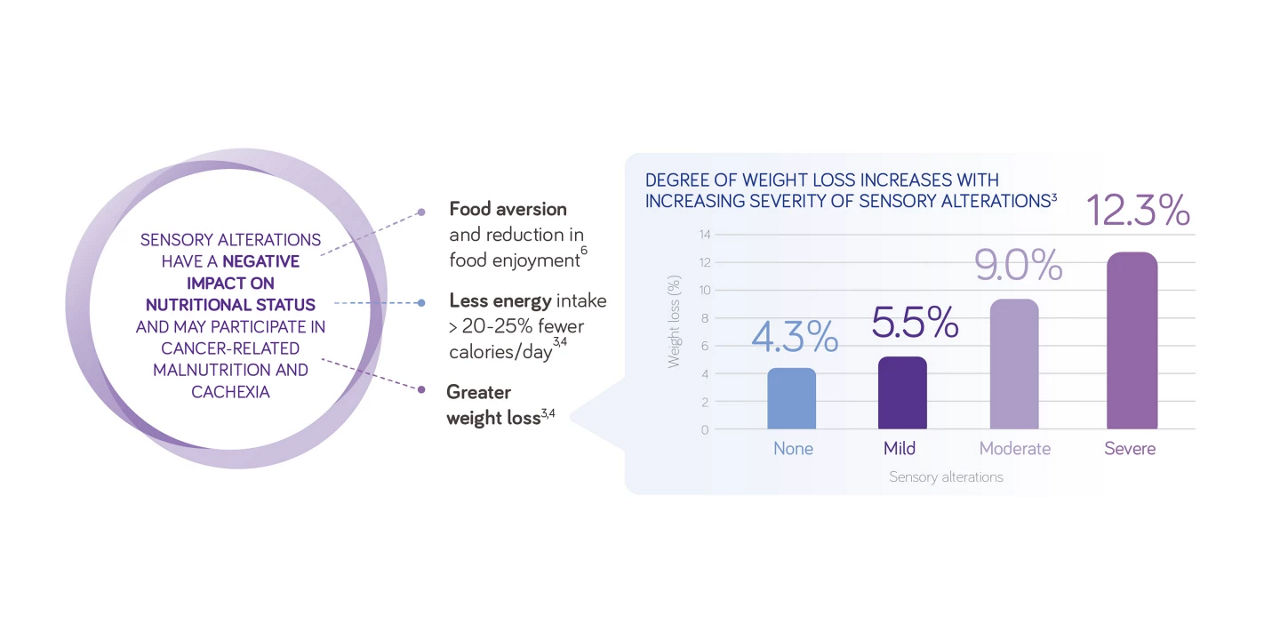
Taste alterations can contribute towards malnutrition2
Taste alterations may develop throughout the course of the cancer, before, during and up to one year after treatment.2 These taste alterations could lead to a reduced intake of food and therefore have a negative impact on the nutritional status with up to 25% reduction in nutritional intake, leading to greater weight loss.3,4
Tastes change, nutritional intake should not
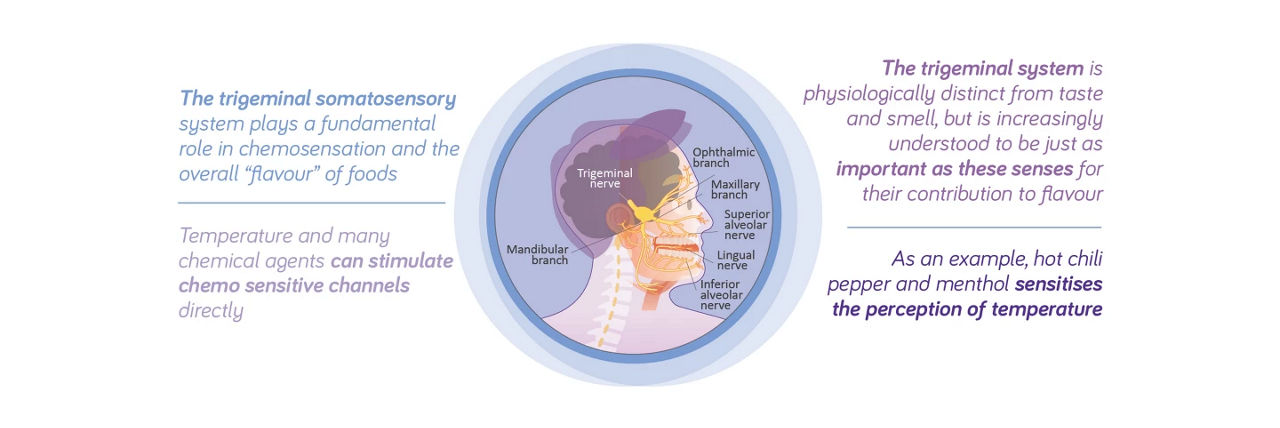
Taste alterations in patients with cancer can vary in character and severity. The trigeminal somatosensory system plays a fundamental role in chemosensation and the overall flavour of foods.5 To compensate for taste alterations, some patients need more intense stimuli, such as adding spice or ginger, while others need less intense flavours.6,7 Nutritional interventions in cancer patients, such as Oral Nutritional Supplements (ONS) can improve overall energy and protein intake, overall body weight, muscle mass and quality of life. Furthermore, adressing the needs of taste alterations and selecting optimal tasting medical nutrition can improve outcomes in patients with cancer.8-12
The Role of the Trigeminal System5
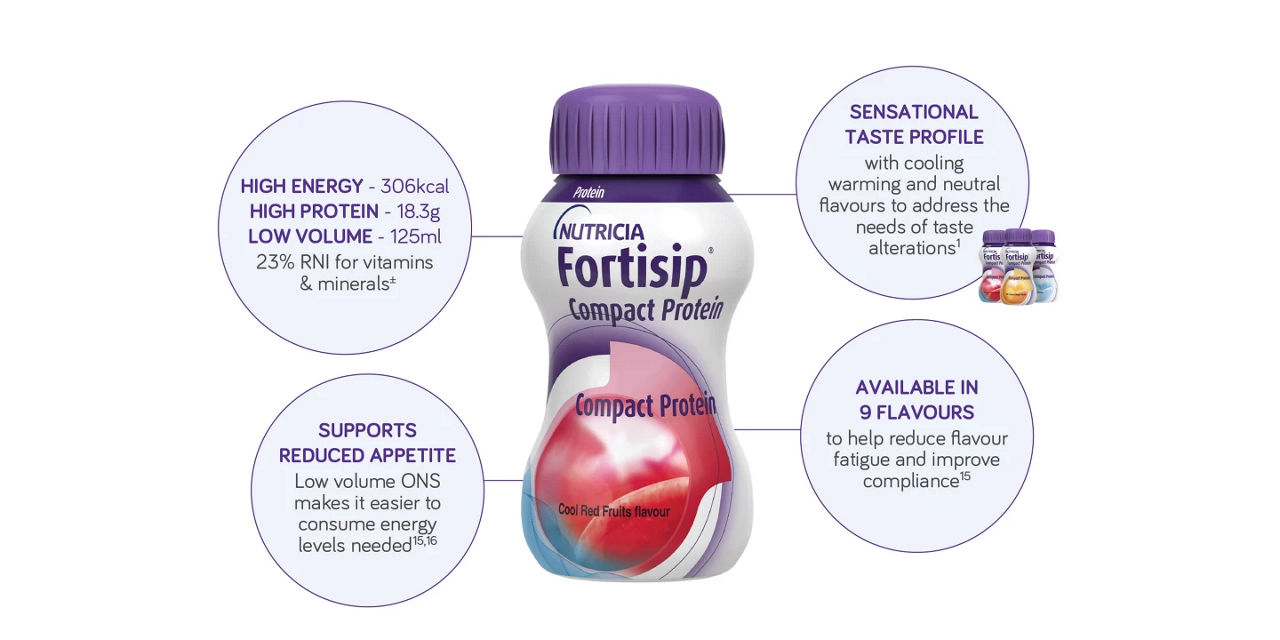
Support nutritional intake of patients with taste alterations with Fortisip Compact Protein cooling, warming and neutral flavours
Fortisip Compact Protein is available in 9 flavours to help prevent flavour fatigue.2Included within this range, are our cooling, warming and neutral flavours, appreciated in patients with cancer1. The sensory adapted flavours address the needs of taste alterations and support with compliance by combating flavour fatigue.15

* Product can be provided to patients upon the request of a healthcare professional.
± RNI (Reference Nutrient Intakes) for males 19-49yrs (excl Na, K, and Cl).
- De Haan et al. Supportive Care Cancer 2021, 29(10):5691–5699.
- Spotten et al. Ann Oncol 2017; 28:969-84
- Brisbois et al. J Pain Symptom Manage. 2011;41(4):673-683.
- Belqaid et al. Acta Oncol 2014; 53:1405-12
- Felix Viana et al. 2011 "Chemosensory Properties of the Trigeminal System"
- Boltong A, et al. Support Care Cancer. 2012;20:2765-74
- De Vries YC, et al. Support Care Cancer. 2016;24:3119-26
- Arends et al. Clin Nutr 2017; 6(1):11-48
- Arends et al. ESMO Open 2021; 6(3):100092
- Kabata et al. Support Care Cancer 2015; 23(2):365-70
- Manásek et al. Klin Onkol 2016; 29(5):351-357
- Garcia et al. Nutr Cancer 2020; 72(5):801-807
- TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades Book
- Tominaga M. (2005). Molecular mechanisms of trigeminal nociception and sensation of pungancy. Chemical senses, 30 Suppl 1, i191-i192.
- Hubbard GP. et al. Clin Nutr. 2012; 31: 293-312
- Hubbard GP. et al. Proc. Nutr. Soc. 2012;69(OCE2):E16